
Sponsored By: Reflect
This essay is brought to you by Reflect, an ultra-fast notes app with an AI assistant built in directly. Simplify your note-taking with Reflect's advanced features, like custom prompts, voice transcription , and the ability to chat with your notes effortlessly. Elevate your productivity and organization with Reflect.
One of my gripes with the technology industry is how billionaires think of themselves as John Galt from Ayn Rand’s Atlas Shrugged. They lionize independence and get grumpy about any form of regulation. This view is, of course, misguided. While technology is the best lever we have to improve human existence, we still need a hand to push down on that lever. Government intervention, properly applied, is how frontier technologies get used. That’s why this brilliant piece by former Figma engineer Jamie Wong struck me so deeply. He explores how we fixed the ozone layer, not with privately built technology alone, but with global partnerships devoted to helping the planet. While we may all enjoy the riches that come from the success of big tech companies, the whole point of this industry is to make people’s lives better. This piece (which is part one of a two-part article) is a reminder about how frontier tech can do that.—Evan Armstrong
In a demonstration at the 1930 meeting of the American Chemical Society, a chemical engineer named Thomas Midgley, Jr. took a deep breath, inhaled a novel compound deep into his lungs, and held it briefly before exhaling and blowing out a candle. It was theatric proof of two key properties of a new product—the stuff was safe to breathe in and wasn’t flammable.
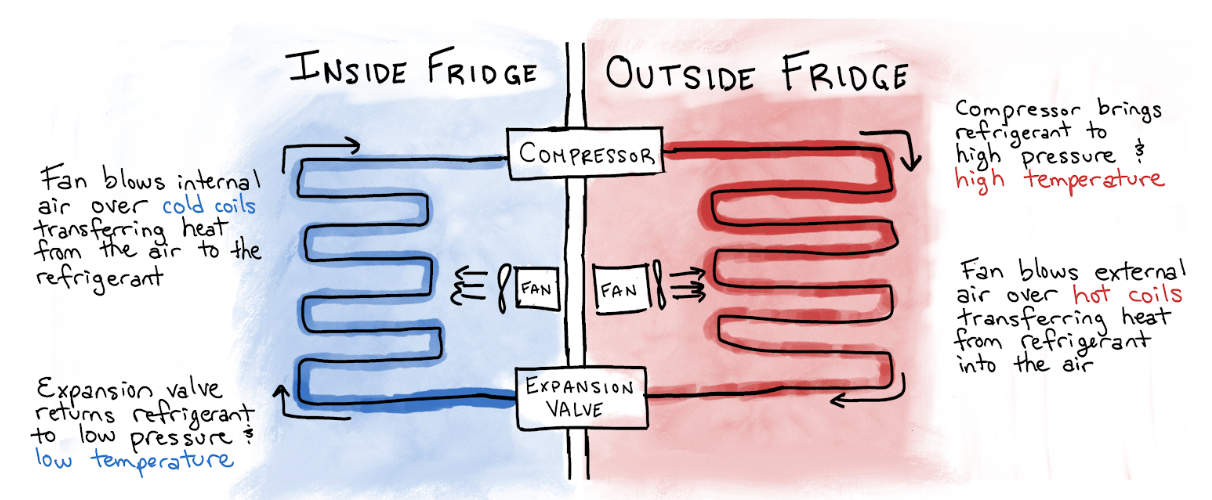
A few years earlier, Midgley had been tasked by the Frigidaire, then part of General Motors, to address a problem holding back widespread adoption of home refrigerators. Electric refrigerators, which had been available for household use since 1915, were becoming financially attainable for many American homeowners, but a key component was giving many homeowners pause: refrigerants, the substances pumped from the inside of the refrigerator to the outside and back in an infinite loop.
Transform the way you take notes with Reflect, the ultra-fast notes app with a baked in AI assistant. Whether you're jotting down ideas or organizing detailed information, Reflect's custom prompts and voice transcription make the process a breeze. You can even chat with your notes, making your workflow more dynamic and efficient. Reflect adapts to your needs, offering unparalleled convenience. Elevate your productivity today.
All illustrations courtesy of the author.
Before Midgley’s discovery, the popular options for refrigerants were plagued by one of two problems. Some, such as ammonia, were toxic, making them unappealing in homes for obvious reasons. Others, including butane, were flammable, rendering them worrisome in a kitchen with a wood- or coal-powered stove.
Through his showy demonstration, Midgley hoped to lay to rest any concerns around flammability and toxicity. The gambit worked.
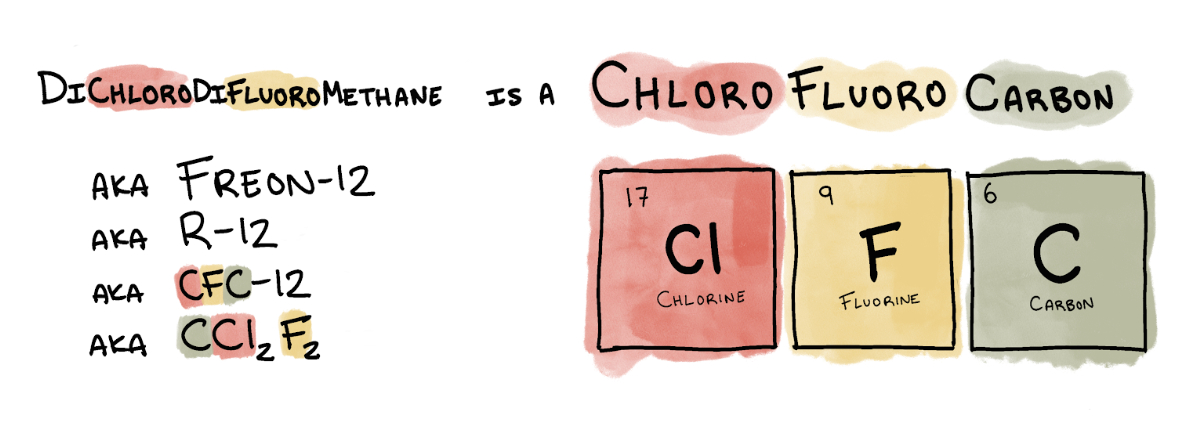
Later that year, General Motors and DuPont jointly formed Kinetic Chemicals and started production of Freon-12 (or R-12), the substance Midgley huffed. It was the first of a class of refrigerants collectively known as chlorofluorocarbons, or CFCs.
Freon quickly became a market leader used in refrigerators and air conditioning units. By the early 1940s, Midgley was one of the most celebrated chemical engineers in the United States, decorated with industry awards. In 1944,14 years after huffing Freon, he was elected president and chairman of the American Chemical Society.
However, that demonstration was not his first.
Finding hope in climate doom
With a never-ending deluge of climate doom in the news, it’s easy to feel jaded or hopeless. Nothing is improving, nobody is doing anything, and there’s nothing I can do.
But, as John Green noted in The Anthropocene Reviewed, "Despair isn't very productive. That's the problem with it. Like a replicating virus, all despair can make is more of itself."
I admit I’m not an optimist by nature, so after I left Figma—where I spent over five years as one of the first engineers—to explore what role I could play in stabilizing our climate, I went in search of optimism.
I wanted to dig beyond the melting glacier narrative and vague we can do it! platitudes. I wanted to see what real solutions looked like and find the people who believe they can personally bend the arc of history.
I read Bill Gates’s How to Avoid a Climate Disaster, Saul Griffith’s Electrify, and Gretchen Bakke’s The Grid—all books focused on honestly assessing where we stand in combating our warming climate, pointing at the technologies ready for deployment, pending research breakthroughs, and those we need to move on from. I listened to the archives of How to Save a Planet and My Climate Journey, two podcasts featuring interviews with people at the forefront of climate solutions. Then I began sheepishly introducing myself to climate tech founders as an angel investor.
In the process of running due diligence before I ultimately wrote my first check to a company called Recoolit, I found two main sources of optimism —one from the public sector’s triumph over the catastrophic outcomes of an unmitigated hole in the ozone layer, and one from the ambitions and progress of Recoolit itself.
Recoolit’s story will come at a later point, but today I’d like to share that first story—the triumphs of scientists and regulators in finding and fixing the ozone hole. I feel compelled to share these stories—to spread that optimism—because narratives stick to our collective consciousness in ways that charts, graphs, and statistics rarely do.
Despite the barrage of climate doom, there are, in fact, success stories from decades of environmental action—ones in which we do steer our home planet away from the proverbial iceberg. We’re just not very good at telling them, because sometimes the solution looks less like the Avengers battling Thanos and more like a woefully dry policy document stipulating the details of a 20-year plan.
The history of the ozone hole offers one of those stories—a near-catastrophe that dominated headlines in the 1980s and ’90s, and then disappeared from public consciousness as we ameliorated the problem.
Not so long ago, the term “ozone hole” evoked a vague feeling of concern, but I had no answers to the most basic questions: What exactly is the ozone hole? What caused it? Why was it such a big deal? What did we do about it? And why don’t we hear about it any more?
In answering those questions, we’ll uncover a century-spanning conflict featuring a Nobel laureate, an Antarctic expedition, and the only international policy ratified by every country on Earth. We’ll take pit stops at all of these curiosities, but for now, let’s start with the man who Georgetown University environmental historian J. R. McNeill said had a "more adverse impact on the atmosphere than any other single organism in Earth's history."
A one-man environmental disaster
In 1924, six years before inhaling Freon, Midgley held a press conference to calm fears about an earlier product he had developed: tetraethyl lead, an additive to gasoline that allowed car engines to run more quietly. The year before, a new chemical plant was proving disastrous for workers’ health. Within the first two months of operation, 12 employees had fallen sick with lead poisoning. Five died.
At the press conference, Midgley poured tetraethyl lead on his hand and breathed the fumes for a full minute. He claimed he could do this every day without consequence.
Midgley knew he was lying. A year before the press conference, as plant workers were getting sick and dying, he took an extended vacation to Miami to try to cure himself of lead poisoning. In a letter to the American Chemical Society explaining why he couldn’t attend a gathering to celebrate his discovery of tetraethyl lead, Midgley wrote, "After about a year's work in organic lead I find that my lungs have been affected and that it is necessary to drop all work and get a large supply of fresh air."
When presented with an inconvenient truth about his hyper-profitable first invention, Midgley was more than happy to bend the truth for personal gain. It’s unclear whether he knew that breathing in CFCs would be equally irresponsible, but that first demonstration showed how little he cared to find out.
Still, it’s somewhat astounding that a man with such blatant disregard for public health would knowingly cause one public health crisis and then maybe accidentally, but equally recklessly, cause another. Midgley had “an instinct for the regrettable that was almost uncanny,” as Bill Bryson put it in A Short History of Nearly Everything.
The perils of Midgley’s second invention—CFCs—wouldn’t be appreciated until nearly 50 years after he blew out the candle.
Detroit, Michigan, 1941. Buying refrigerators at the Crowley-Milner department store. Photograph by Arthur S. Seigel (Library of Congress).
A prediction about the end of the world
Years, then decades, passed with an explosion in sales of air conditioners and refrigerators. They moved from being technological curiosities to household staples. From 1930 to 1970, adoption of refrigerators in American homes rose from 13 to 99.8 percent, constituting hundreds of millions of sales. Unbeknownst to millions of homeowners, this cooling hardware was teeing up an environmental disaster that would penetrate the global consciousness.
It took a chain of discoveries stretching over two decades to confirm the threat. The first domino was toppled by a scientific paper that pointed out that every molecule of CFC ever produced had stayed in the atmosphere.
When University of California, Irvine professor Frank Rowland read this paper, alarm bells rang in his head. As an atmospheric chemist, he realized that CFCs, which were lighter than air, would drift higher and higher in the atmosphere until they found themselves in the ozone layer—a layer of the stratosphere rich in ozone, a gas whose molecular form contains three oxygen atoms instead of the regular two. The ozone layer protects earth from the full brunt of the sun’s ultraviolet rays.
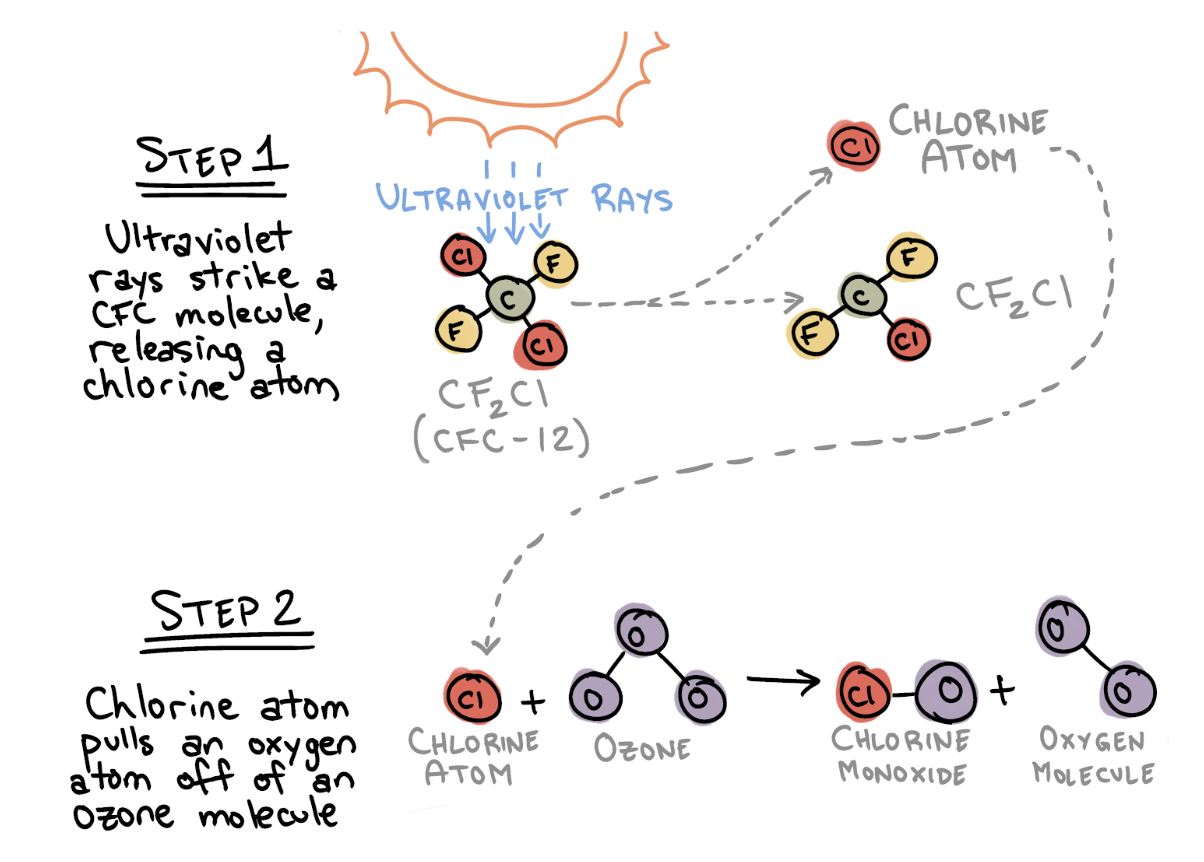
Rowland had an intuition that the presence of CFCs in the ozone layer spelled trouble. At high altitudes, one molecule of CFC would decompose when hit by ultraviolet light, freeing an atom of chlorine. The chlorine atom would then tear an oxygen atom off an ozone molecule, effectively destroying that ozone molecule.
This two-step reaction alone wasn’t cause for alarm. If every molecule of CFC ever produced destroyed one molecule of ozone, the effect on the ozone layer would be negligible.
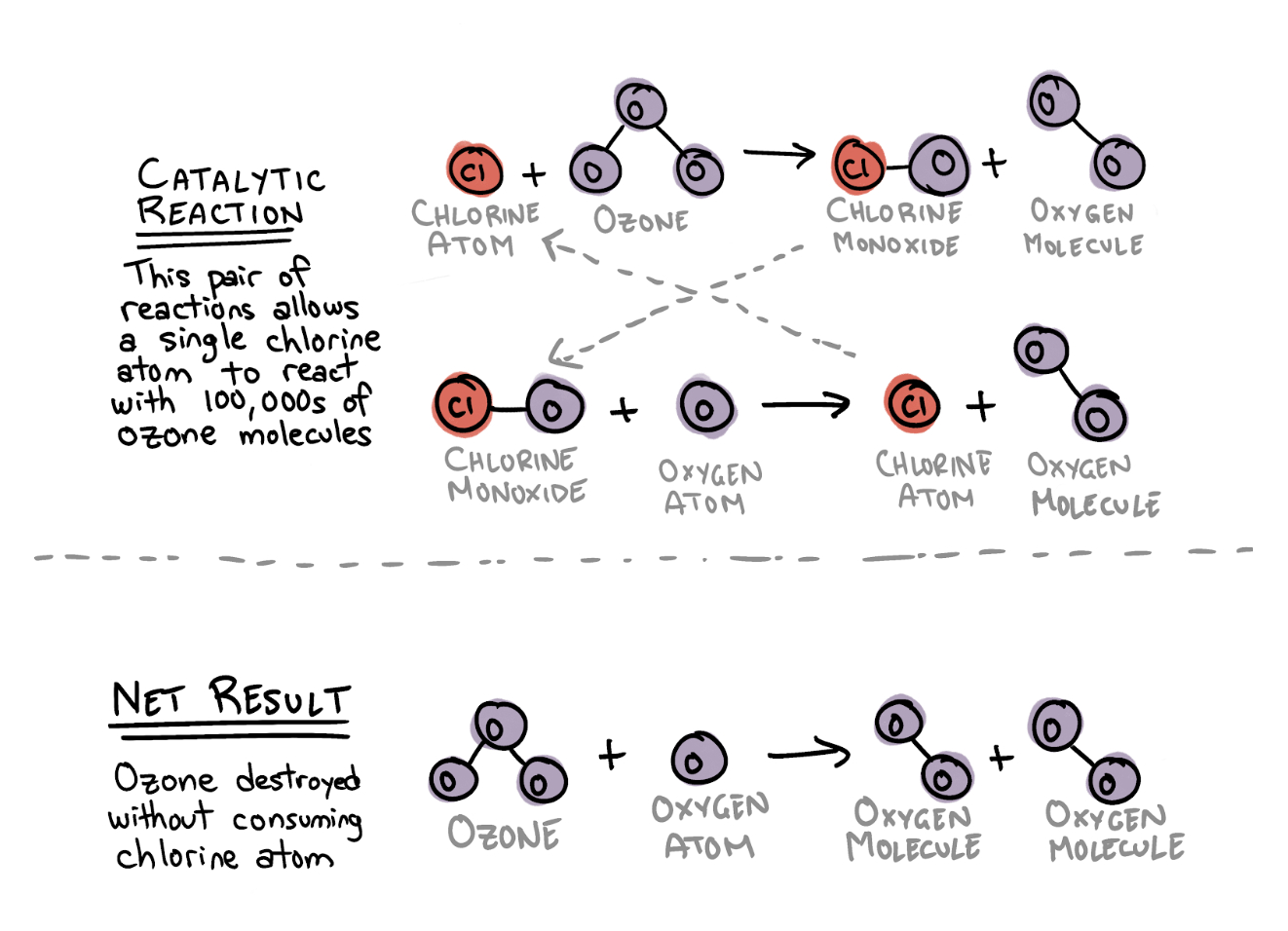
But Rowland believed that a single molecule of CFC might be far more destructive than that. He theorized that the chlorine atom released by the CFC would act as a catalyst: It would facilitate a series of chemical reactions without being consumed by them.
When Rowland did the math, his estimates were horrifying. Rather than a molecule of CFC destroying a single molecule of ozone, it had the potential to destroy hundreds of thousands. At predicted concentrations of CFCs in the coming decades, this would be disastrous for the ozone layer.
When Rowland’s wife asked about his work one night in 1973, he responded, “It’s going very well. It just means, I think, the end of the world.”
In September of 1974, 44 years after Midgley’s stunt inhaling Freon, it was now Rowland’s turn to cause a CFC-related stir at a meeting of the American Chemical Society. Having just published his findings in the scientific journal Nature, he and his colleague Mario J. Molina were emboldened to sound the alarm.
They proposed that CFCs would eventually cause a depletion of the ozone layer—a premise that came to be known as the Rowland-Molina hypothesis. They warned that 10 percent of the ozone could be lost in the next 50 years.
At the time, CFCs were predominantly used in aerosols rather than refrigerants. While the refrigerant-centric story of CFCs is both the larger and longer arc, it’s worth a brief aside about aerosols to chart the interactions between scientists, journalists, regulators, and manufacturers.
The press took notice of Rowland’s warning. On September 26, 1974, the New York Times ran an article titled “Tests Show Aerosol Gases May Pose Threat to Earth”: "The most prevalent concern, however, is not for total loss of the ozone, which is broken down and restored in a complex sequence of day and night chemical reactions. Rather, it is a fear of sufficient depletion to cause widespread skin cancer and other effects."
On June 30, 1975, as public concern was mounting, DuPont ran a full-page ad in response. The company promised to “stop production of the offending compounds” if evidence showed that CFCs’ impact on the ozone layer posed serious health concerns.
At this point in time, the Rowland-Molina hypothesis was still just that: a hypothesis. There wasn’t yet any empirical evidence of widespread ozone depletion. Despite that, product manufacturers decided to play it safe when it came to use cases with obvious alternatives. Why risk consumer panic when perfectly good alternatives were available?
Deodorant companies largely switched from spray cans using CFCs as the propellant to roll-on alternatives, and home cleaning aerosol cans gave way to the pump-action variety we use today in bottles of Windex. When the still-new U.S. Environmental Protection Agency (EPA) banned CFCs in aerosol spray products in 1978, usage had already declined more than 40 percent due to changes in manufacturer and consumer preferences.
For CFCs’ application as refrigerants, however, the action was not so swift. Unlike in aerosol sprays, there were no simple drop-in replacements in air conditioners and fridges. For appliance manufacturers to change their choice of refrigerant, and for DuPont to cancel one of its largest product lines, it would take iron-clad proof of a real ozone problem.
That proof was destined to emerge from, of all places, Antarctica.
A frigid expedition
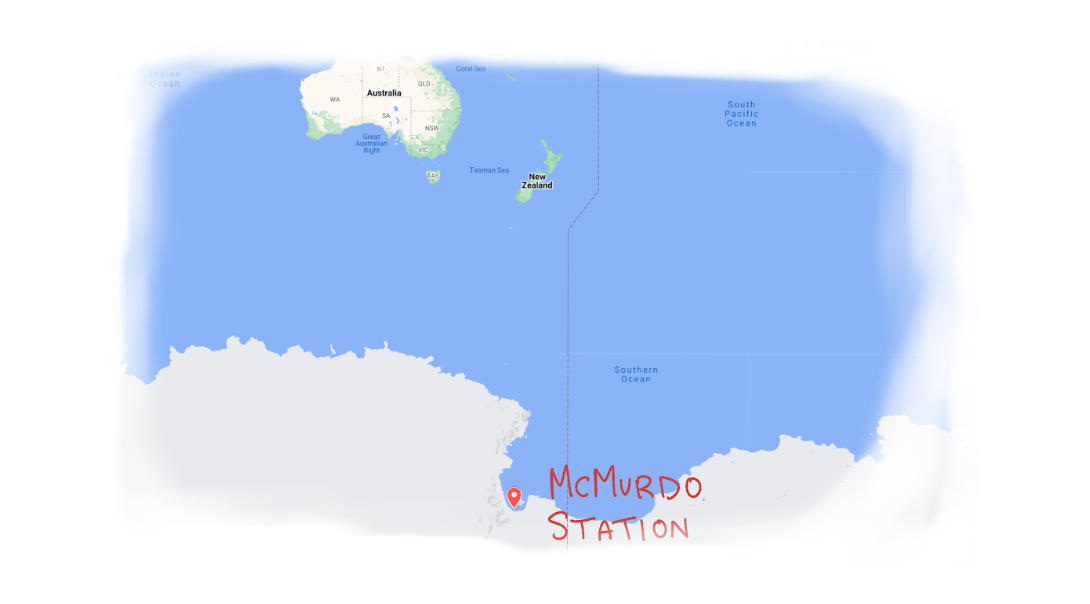
Dr. Susan Solomon, 1987. Source: National Oceanic and Atmospheric Administration.The main goal was getting out without frostbite, said scientist Susan Solomon, reflecting on the expedition she led in 1986 to McMurdo Research Station in Antarctica. Solomon and her team ventured far south to investigate a manifestation of Rowland’s prediction: a gigantic hole in the ozone layer hovering above the South Pole.
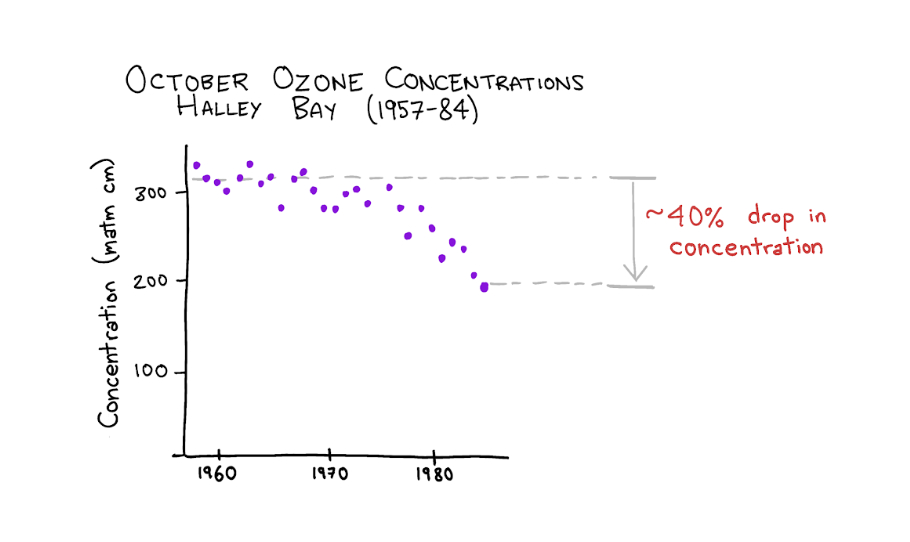
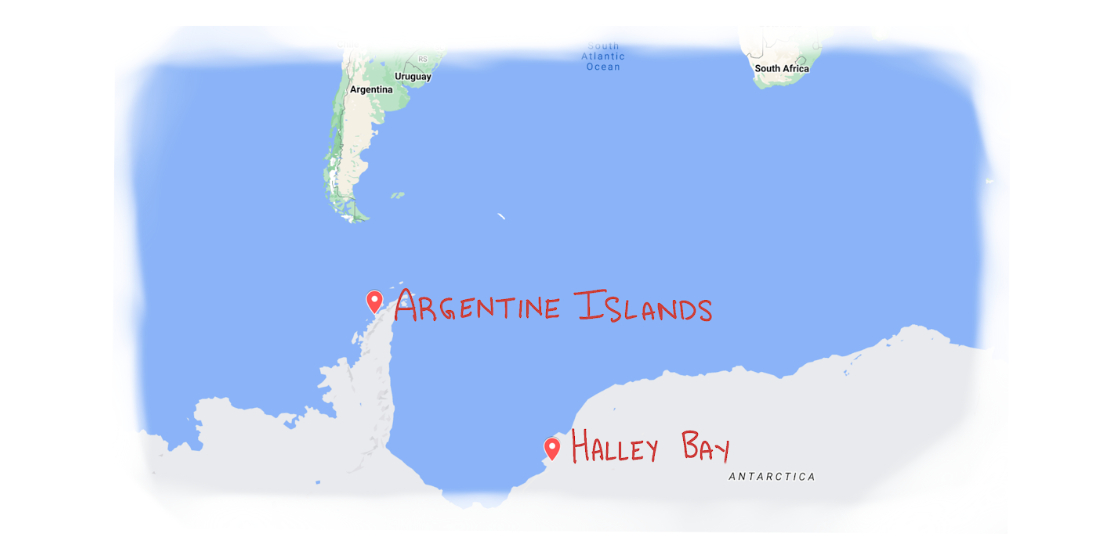
Solomon’s expedition was funded by the U.S. National Science Foundation following a series of startling scientific discoveries from the previous two years.The first empirical evidence of trouble came in 1985 from a group of British scientists studying the changing chemical composition of the atmosphere in Halley Bay, Antarctica. In the air column directly over their research station, they found a 40 percent decrease in ozone concentrations.
Based on data from Farman et. al, 1985.The researchers pointed out that the problem appeared to be much less severe at another Antarctic research station further north on the Argentine Islands. Why was there such a discrepancy?
To add to the puzzle, NASA’s research satellite, the Nimbus 7, showed no ozone depletion at all. The Nimbus 7 had been gathering data for years before, so why hadn’t the hole already been detected?After agonizing over the problem, NASA scientist Richard Stolarski eventually solved the conundrum. By examining the satellite’s processing procedure, he realized that the Nimbus software had been written to discount data below a certain numerical value, assuming that such figures were the result of equipment failures or software bugs.
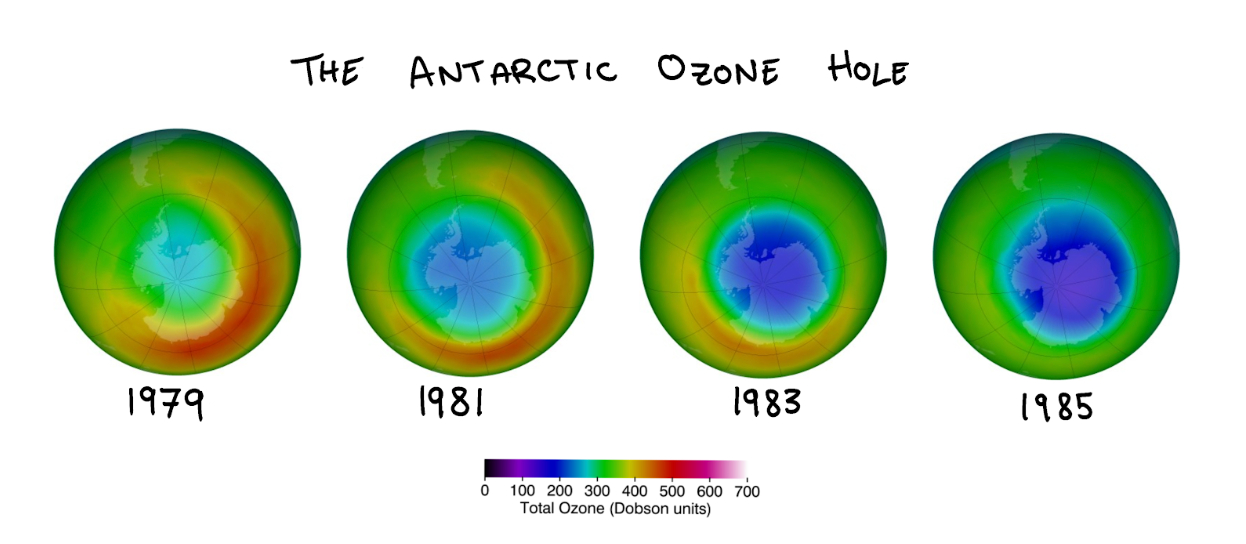
The ozone numbers had gotten so alarmingly low that the Nimbus had been ignoring them, assuming that they could not possibly be real. After changing the threshold of allowable values, the satellite data confirmed not only the existence of a hole in the ozone layer, but its geographical scope.
The hole covered nearly all of Antarctica. It was the size of the continental United States.
Measurements from October of respective years. Source: NASA Ozone Watch.
The urgent question now was, How do we stop the hole from growing? To answer that, we needed to know why the hole was there in the first place. While Rowland’s CFC theory was still a leading contender, DuPont wasn’t going to phase out a lucrative product line without indisputable evidence.
Enter Susan Solomon aboard a ship en route to the Antarctic with four research groups in tow. They went in search of data to support or refute the CFC theory and its alternatives. Once on site at the bone-chilling polar desert, the teams set to work running experiments.
After releasing a weather balloon to carefully measure ozone concentrations at varying altitudes and standing on roofs in the blistering cold to measure atmospheric concentrations of chlorine-dioxide, the researchers had the data they needed.
Solomon and her team held a press conference to announce their preliminary results: The data strongly supported the CFC theory.
With the cause clear, it was time to pass the torch to policymakers.
The ‘Ray-Ban Plan’
Confronted with public demands to address the reality of the ozone hole caused by CFC emissions, regulation was the obvious choice. But it was the mid-1980s, the prime years of Ronald Reagan’s presidency. It’s an understatement to say that as president, Reagan had a penchant for deregulation. By 1986, the Reagan administration had eliminated nearly half of all federal regulations that existed in 1981.
When presented with evidence of the ozone crisis, rather than encouraging regulation, Donald Hodel, Reagan’s interior secretary, recommended “personal protection” in the form of hats and long-sleeved shirts.
The press did not treat this suggestion charitably. Cartoonist Herbert Block offered a visual interpretation in a 1987 edition of the Washington Post:
Published by the Washington Post on June 3, 1987. Cartoon by Herbert Block. (Source)
As a nod to the sunglasses manufacturer, the advice eventually came to be known as the “Ray-Ban Plan.” A spokesperson for a New York-based sunglasses distributor told the Los Angeles Times, “Sounds fine with us… We’ll be happy to set the Interior secretary up as a distributor of Ray-Ban sunglasses.”
Thankfully, the story of global response to the ozone hole does not end with a snarky cartoon.
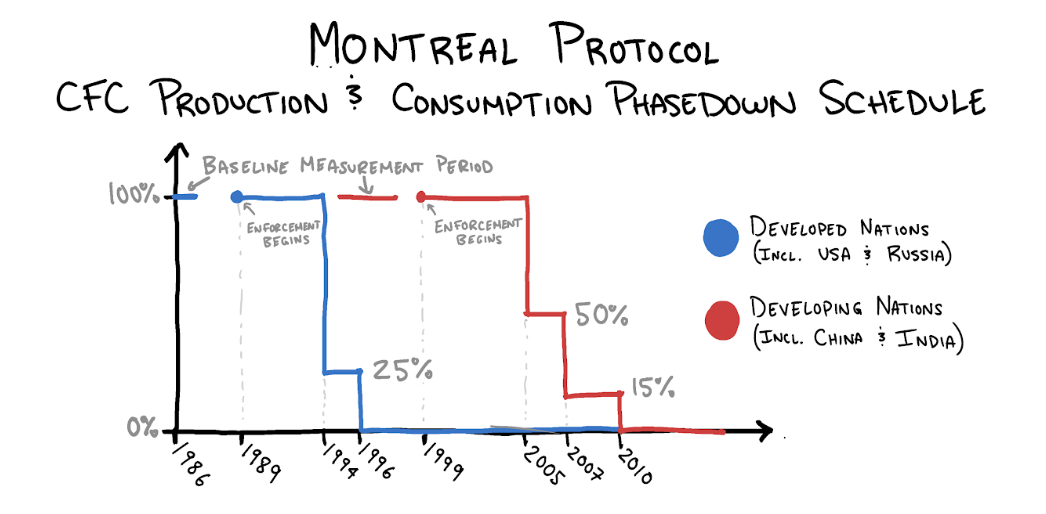
The Montreal Protocol
The closest thing to an "Avengers assemble!" moment in this story was a 1987 conference in Montreal packed with diplomats hailing from 46 countries. The delegates went to Canada to negotiate an agreement for the phase-out of “ozone depleting substances”—chiefly CFCs.
Given the way debates over global climate policy have gone in recent decades, you might understandably assume that what came next was a disaster—years and years of endless vague discussion and aspirational messages, with politicians stonewalling or dragging their feet.
You would be wrong. On September 16, 1987, after 18 months, negotiations concluded. The Montreal Protocol, as it came to be known, laid out specific timelines for reducing consumption and production of CFCs. Delegates returned to their home nations to convince their respective governments to ratify the agreement and allow it to come into enactment. They succeeded.
Despite hesitation about regulation from the Reagan administration, when the decision to ratify the Montreal Protocol was brought to the United States Congress, it was approved unanimously by the Senate, 83-0. By the end of 1988, 30 countries had ratified the treaty, including the eight wealthiest in the world. When East Timor signed in 2009, the document became the first environmental pact to be signed by all 196 member states of the United Nations.
Source: The UN Ozone Secretariat.
But despite producing an agreement that former UN Secretary General Kofi Annan referred to as “perhaps the single most successful international agreement to date,” the conference itself was decidedly un-cinematic—so much so that the Wikipedia page for the resulting Montreal protocol contains no description of events during negotiations. Even the man given the title of “the Father of the Montreal Protocol,” Egyptian scientist and UN environmental chief Mostafa Tolba, is given only a single unassuming sentence.
I wish there were a scene—a daring negotiation gambit, fisticuffs in Montreal, a dramatically exposed corrupt politician, something to help us glue this monumental moment in our collective consciousness. I want source material for a Lin-Manuel Miranda production or a Christopher Nolan biopic. Hell, I’d even take a clever quip jotted down by a journalist.
It feels almost cosmically cruel that the origin and discovery of the ozone problem offer scenes, and the solution offers none. Thomas Midgley Jr. inhaling CFC for an audience. Franklin Rowland describing his research as both “going very well” and signifying “the end of the world.” Susan Solomon’s team in the Antarctic standing on roofs and mingling with penguins. Cartoonist Herbert Block sketching plants and cows wearing sunglasses in response to the Reagan administration’s “Ray-Ban Plan.” Yet it all culminates in the undramatic negotiation of a document so dry I fell asleep reading it despite months of adjacent research.
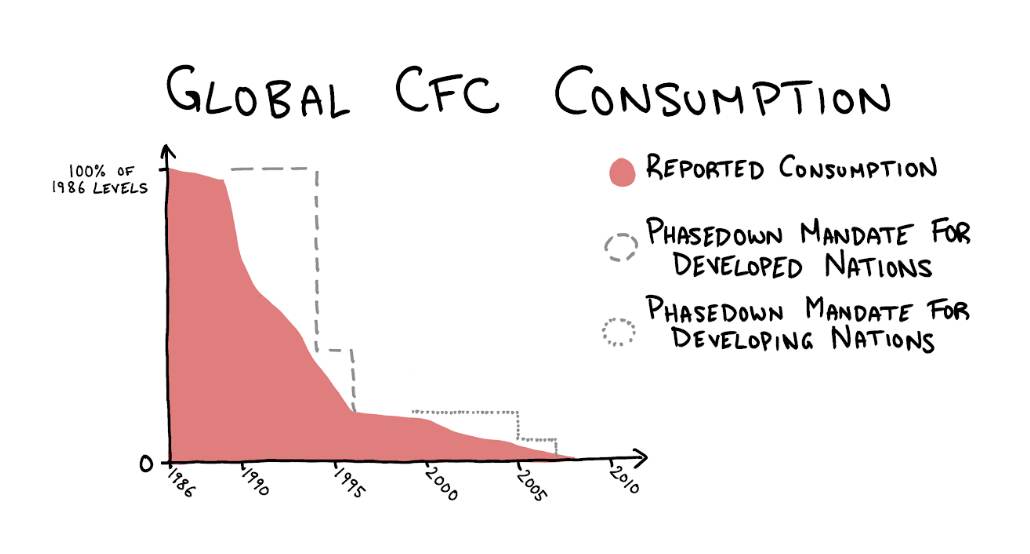
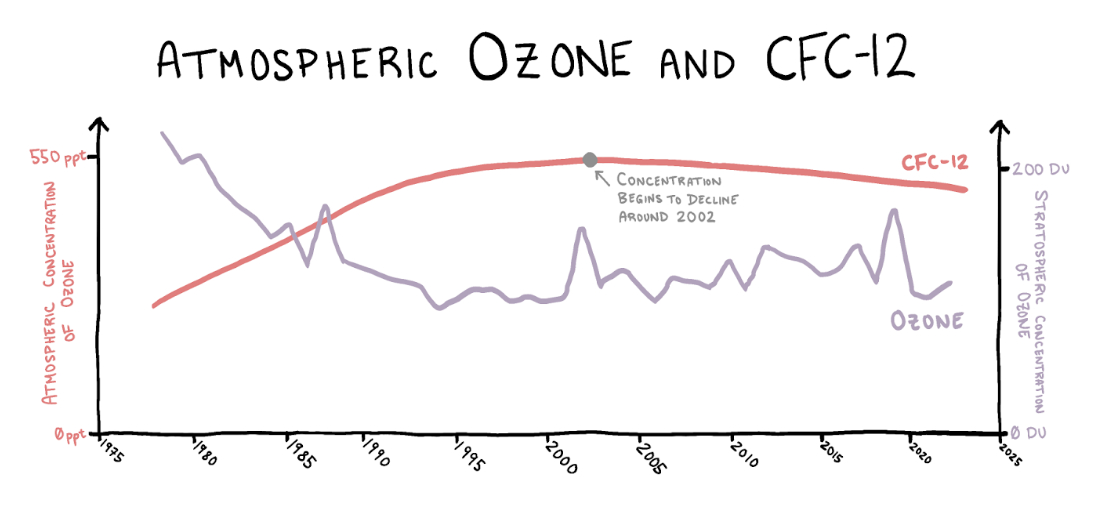
This pains me because I want the Montreal Protocol to be firmly embedded in our memories. Because it deserves a place in our memories. The nations of the world came together, heeded the warning of the scientific community, made a binding agreement, and the agreement worked. Since the ratification of the Montreal Protocol, new emissions of CFCs—as measured by changing atmospheric concentrations—have dropped nearly to zero, a reduction of more than 99 percent. Compared to the direct impact of global climate change policy like the 1997 Kyoto Protocol (which the U.S. refused to ratify and placed no binding reduction targets on India or China), this is a miracle.
The ozone hole in Antarctica is still there—but, crucially, it stopped getting worse. CFCs stay in the atmosphere for 50 to 150 years, so emissions from long ago will continue destroying ozone for years to come, but nevertheless the progress is promising. Atmospheric CFC concentrations are slowly dropping, and scientists predict that global ozone concentration will return to 1980 levels by 2040.
Source: Our World in Data.
Sources: CFC-12 data from NOAA Global Monitoring Lab. Ozone data from NASA Ozone Watch.
DuPont finally made good on its 1975 promise “to stop production of the offending compounds.” On March 24, 1988, 10 days after Congress ratified the Montreal Protocol, the company proclaimed, “DuPont sets as its goal an orderly transition to the total phase-out.” This statement came only three weeks after DuPont’s chairman claimed that “at the moment, scientific evidence does not point to the need for dramatic CFC emission reductions.” At the time, DuPont produced about a quarter of the global supply of CFCs to the tune of $600 million of annual revenue.
The scientists of this story received the recognition they deserved. In a 1995 ceremony in Stockholm, Roland and Molina received the Nobel Prize in Chemistry "for their work in atmospheric chemistry, particularly concerning the formation and decomposition of ozone.” In a 2000 ceremony in Washington, President Clinton presented Solomon with the National Medal of Science “for exemplary service to worldwide public policy decisions and to the American public.”
The ozone story has a happy ending, both for the protagonists and for the general public. I just wish the world knew about the happy ending—especially because there’s a sequel whose happy ending isn’t yet written. Refrigerants didn’t disappear. To get around the Montreal Protocol, companies developed new versions of the chemicals—in other words, CFCs evolved.
While the successors addressed CFCs’ cardinal sin of ozone depletion, they joined CFCs as major contributors to the most present environmental issue of our time: climate change. But that’s a story for another day.
Subscribe to read part two, in which a few pages of a book send a Silicon Valley tech worker to Jakarta in search of refrigerants to destroy.
Jamie Wong is a writer, software engineer, and angel investor living in San Francisco. Previously an early software engineer at Figma and Khan Academy, Jamie is currently a member of South Park Commons. You can read more of his writing on his personal website.
To read more essays like this, subscribe to Every, and follow us on X at @every and on LinkedIn.
The Only Subscription
You Need to
Stay at the
Edge of AI
The essential toolkit for those shaping the future
"This might be the best value you
can get from an AI subscription."
- Jay S.
Join 100,000+ leaders, builders, and innovators

Email address
Already have an account? Sign in
What is included in a subscription?
Daily insights from AI pioneers + early access to powerful AI tools





Comments
Don't have an account? Sign up!
What an amazing article. Thank you.🙏