
Batteries don't get the credit they deserve. They're hidden inside gadgets, making it easy to forget the huge role they've played in helping us harness electricity. Believe it or not, batteries were our first source of electricity before we even had any idea what electricity was (let alone how to build electric grids and infrastructure).
The first battery was invented in the early 1800s and has changed our world since then. It paved the way for many technological advancements. Without the ability to store energy for long periods of time, smartphones, laptops, and long-range electric vehicles wouldn’t be possible.
Looking to the future, battery technology could play a huge role in reducing our use of fossil fuels. Sources like solar panels and wind turbines need to save all that weather-dependent energy somewhere. Unfortunately, battery components are made of limited materials that aren't easy to recycle. As a result, they may wind up being the bottleneck to achieving sustainable and abundant energy—that’s why efforts to study and evolve them are so important.
Read on to learn more about how batteries were invented, what’s happening today in battery innovation, and where the technology needs to go for a renewable energy future.
The History of the Battery
Batteries got their start in a rather odd way. In 1786, an Italian physician and biologist named Luigi Galvani was dissecting a frog. When he placed his scalpel to the frog’s leg, he was surprised to find the muscles came to life and the entire limb began to twitch. Earlier in his career, Galvani discovered that nerve impulses were electrochemical in nature.
At the time, the scientific community was only in the very early innings of discovering the true nature of electricity. One important milestone was Benjamin Franklin’s realization that lightning and static electricity were of the same medium. The discovery made Franklin quite a celebrity in European scientific circles, even if his dangerous experiments made him a rather suspect character at home in America.
Despite these advancements, we still didn’t understand exactly what electricity was (or how to produce it). It was one of the biggest scientific questions of the era. Thus, when Galvani’s scalpel induced an electrochemical impulse in the frog leg, a lightbulb went off for him.
Galvani thought electricity was a substance inside the frog triggered by the metal scalpel. He published these findings in a 1791 paper called “Commentary on the Effect of Electricity on Muscular Motion,” which was circulated amongst the scientists of the day. Eventually, his paper made its way to a professor of physics named Alessandro Volta.
The science behind frog legs (and batteries)
Volta was unconvinced by Galvani’s explanation—he thought it highly improbable that the source of electricity was a latent fluid trapped inside biological tissue. After re-creating Galvani’s experiment, Volta realized that the frog leg was not the true source of the electricity, but rather a conduit for it.
The real source of electricity came from the interaction of two disparate metals, the scalpel, and a metal plate, being put into close proximity. The metal plate holding the frog transferred its electrons through the frog leg to the metal in the scalpel. This exchange of electrons created a current which interacted with the frog’s nerves, making its legs twitch.
Volta wanted to test this hypothesis without using a frog as a conduit. He took two coins made of different metals and placed them on either side of his tongue. Instantly, he could feel a tinge of electric current pass between them. Next, he took plates of silver and zinc, separated by cardboard soaked in salt water, and stacked them. With one finger touching the plate of zinc at the bottom and another finger touching the plate of silver at the top, Volta could feel a tiny electric shock.
The ‘voltaic pile’ he erected produced an electrical current…it was the first battery ever made.
Volta’s invention predated knowledge of the atomic structure, so he merely chalked the production of electricity up to some chemical reaction created by the interaction of different metals. This wasn’t far off from the truth.
Converting chemical energy into electric energy
All atoms are inherently endowed with electric energy because they’re composed of charged particles, including protons and electrons. Today, we know that an atom’s structure determines how much electrochemical potential it has.
Atoms that have a complete outer shell of electrons, like the noble gases, are typically very stable and non-reactive. These elements have low electrochemical potential. Atoms with incomplete electron shells are the opposite. They have more electrochemical potential because they want to react with surrounding atoms to reach a more stable structure. In Volta’s battery pile, zinc atoms were more reactive and willing to give up electrons, whereas the silver atoms were the opposite.
Volta found the key to creating an electric current inside the voltaic pile. He placed a solution that could shuttle these electrons between the two metals (the salt-water-soaked cardboard). With some experimentation, he noted that the strength of the current could be increased by stacking a larger pile of zinc and silver plates.
This structure is the basis of all batteries: a reactive material willing to give up electrons on one end (called an anode), and a material willing to accept electrons on the other end (a cathode). The trick is to get the electrons from the anode to flow to the cathode, thus creating a current.
The issue is that the atoms in the anode and cathode are locked in solid structures, meaning they can’t move freely. To solve this, a solution called an electrolyte is placed as an intermediary between the two. It contains free-floating ions that act as a shuttle for electrons, picking them up at the anode end and delivering them to the cathode end.
Volta’s battery was the first way we learned to convert chemical energy directly into electric energy. That’s how the battery became known as a reliable source of electricity before we even knew what electricity was. Predating the energy grid and power stations, batteries were fueling all kinds of early electric devices (from the first electric motors to the telegraph).
Early ideas for electrifying houses: Battery “milkmen” and more
The only issue was that early batteries were pretty inconvenient. They looked like jars with two metal rods stuck inside, the anode and the cathode, and were filled with a liquid, acidic electrolyte. By connecting a wire to the cathode end, you could power anything from a doorbell to a lightbulb, but when all the electrolytes in the solution were used up, the battery would stop producing a current. Then, you would have to service the battery by dumping out the acid and refilling the jar with more electrolyte solution.
Being a ‘battery man’ was a real occupation at the time. Every major telegraph company, like Western Union, hired people whose sole job was to maintain and service the company’s massive, gurgling, acidic batteries powering the telegraph network.
In his book “The Battery,'' Henry Schlesinger writes about the debates that early engineers had regarding what kind of infrastructure might be required to electrify households. It wasn’t yet clear that each country would build a massive electrical grid connecting every home to giant power stations.
Instead, many assumed each home would need their own batteries, refilled on a regular basis (much like milkmen used to deliver bottles of milk). It wasn’t a stretch of the imagination to think households would put out their empty battery jars every couple of days to get a fresh supply.
Another strange, creative idea engineers floated was to install a giant subterranean battery under each home. Its electrolyte could be dispensed and replenished in the same way that rural homes handle the contents of their septic tanks. The process of draining and refilling these batteries with corrosive, liquid substances was not ideal.
The other problem was that early batteries weren’t rechargeable. The electrolyte was producing irreversible chemical reactions with the anode and cathode material. Eventually, reactions would cease, no matter how many times the electrolyte was refilled. Chemists had a hunch that it might be possible to create a battery that generated an electric current through a reversible chemical reaction.
In theory, after depleting a battery you could apply a current to the cathode end to reverse the reaction, thus “recharging” the battery.
The first such design was created by Gaston Planté in 1859. He spent thirty years developing a rechargeable battery, and ultimately accomplished it with lead electrodes and an acidic electrolyte intermediary. When the battery was depleted, adding a current to the cathode end would, over time, recharge it and allow it to function again.
Newfangled electric power spurred the consumer to accept all kinds of new electric gadgets into their home with great excitement. Still, the liquid, corrosive electrolytes powering the batteries were unwieldy and dangerous.
No one wanted a box of acid sitting next to their telephones. To solve that dilemma, in 1888 a “dry cell” was invented by mixing the electrolyte with a paste. It created a kind of gel which sat between the anode and cathode parts. Initial versions of the dry cell were non-rechargeable.
Despite that, they were so useful and compact that they were standardized and featured in all kinds of consumer devices ranging from toothbrushes to children’s toys. Today’s Duracell batteries are still manufactured using the same dry cell model from the late 19th century.
Lithium makes its big debut
The final evolution in battery design came from a yearning to complete the trifecta of rechargeable, high-power, long-lasting batteries. Various types of battery chemistries were developed, like nickel-cadmium, which could supply enough juice to work a high-power laptop. But these were toxic and would die out quickly if you recharge them before they were fully depleted.
The solution to all these problems and more was lithium. Lithium is the smallest atomic metal that exists. It has only two protons, so it’s incredibly light, and just one electron in its outer shell, so it’s very reactive.
Lithium’s natural reactivity made it an incredible chemical candidate in battery production. But its natural instability also made it volatile and prone to exploding or catching fire (whenever pure lithium came into contact with other substances).
Though the US was one of the first to study lithium batteries, ultimately the scientists at Sony and the Asahi Chemical Company figured out a workable design around the 1990s. In their model, rather than using lithium as a pure anode, lithium-ion atoms would play the role of the electrolyte. To generate a current, they would pick up electrons from a porous but stable anode end, then move across a semipermeable membrane over to the cathode end.
The remarkable part of this technology was that these reactions were reversible, which meant these batteries were fully rechargeable! In terms of energy density and life span, the lithium-ion battery was superior in nearly every way from the batteries of yore.
It has been the reigning product in the market since the early 2000s. Its suite of convenient characteristics almost single-handedly enabled our generation to have access to portable and powerful electronic devices, e.g. your smartphone would not have been possible without it.
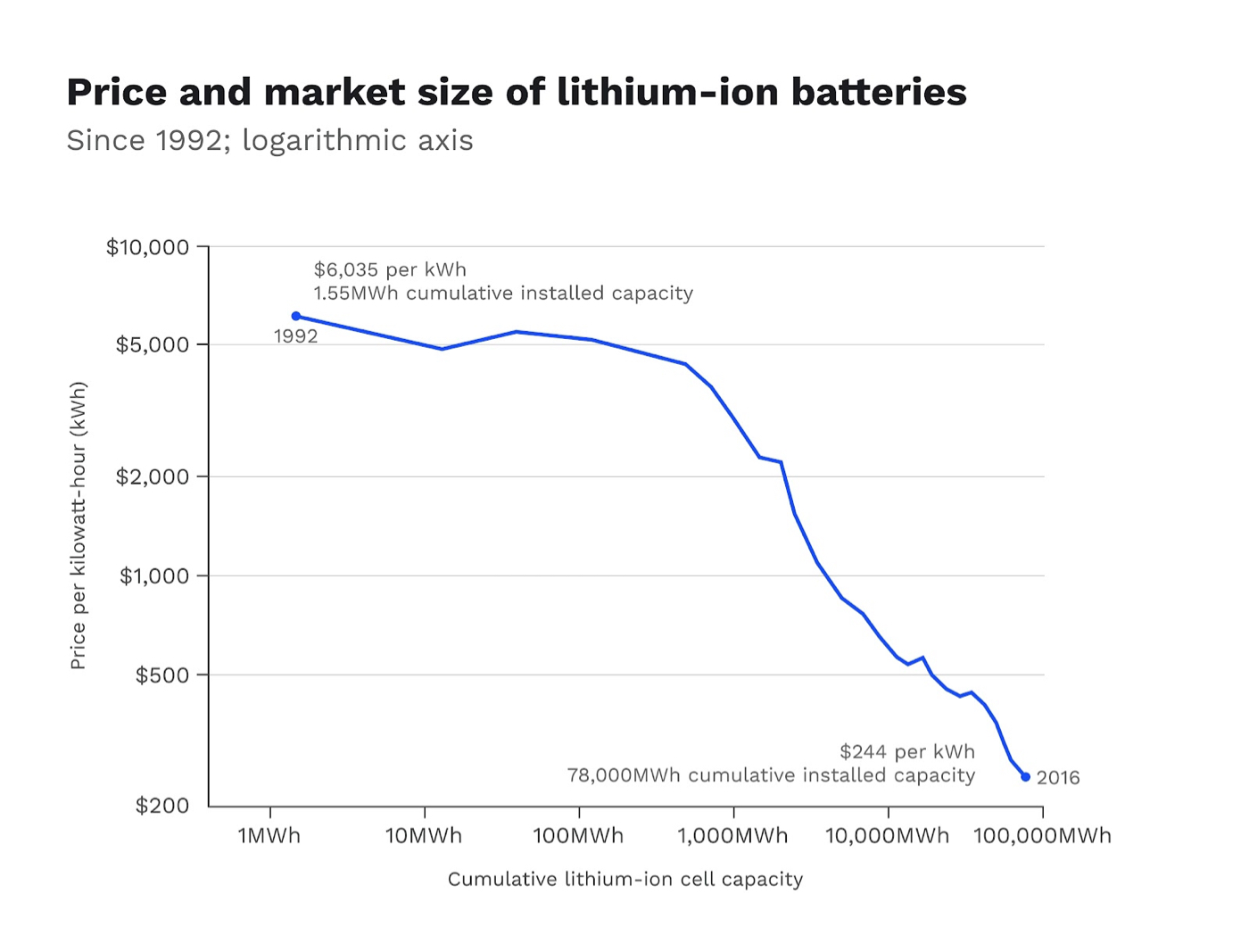
Source: Energy & Environmental Science
The lithium-ion (li-ion) battery was developed in the late 1990s by Sony and the Asahi Chemical Company. Since then, its cost has continued to drop dramatically. The price per kilowatt-hour (kWh) fell from $6,035 per hour in 1992 to $244 per hour in 2016. Common consumer gadgets like laptops are made possible by the affordability of lithium-ion batteries.
Nothing shows the full capability of lithium-ion batteries better than today’s electric vehicles. When the Tesla Roadster was unveiled in 2006 by Martin Eberhard, it was the first electric vehicle on the market that used lithium-ion batteries. At the time, other electric or hybrid brands like the Prius were using a nickel-metal hydride chemistry which had shorter life cycles and weighed more.
Teslas became the fastest cars in the world with lithium-ion technology. Its initial design packed 6,831 individual lithium-ion battery cells — which look like regular Duracells on the outside — together. It produced a 900-pound system that could bring the car from 0-60 mph in just four seconds. (These days, it makes that same acceleration in just 2.3 seconds.)
There’s still more automotive juice to be squeezed out of lithium-ion innovation. Panasonic is one of the most advanced battery manufacturers in the world and has been cooperating with Tesla on battery design since its early years. They’re now very close to releasing an even more energy-dense lithium-ion cell, which will be able to store 900 watt-hours per liter over the current top-of-the-line battery, which has a maximum energy density of 750 watt-hours per liter.
While this would be an impressive improvement, there is a sense that lithium-ion batteries in their current design are entering a world of incremental gains.
Compare and contrast: Batteries, coal, and gas
Ultimately, batteries are evaluated on five characteristics:
- Energy density — a measure of how much total charge a battery can store
- Power density — a measure of the maximum power a battery can output at any given time
- Safety — how easily batteries can short circuit, or how toxic their internal chemistry might be
- Cycle life — how many times you can recharge your battery before it becomes unusable
- Cost
Each battery chemistry has its own particular mix of strengths within this matrix. The ideal battery, of course, would rank highly across all of them — and in that regard, lithium-ion comes very close. It’s incredibly energy dense, relatively safe, has a long cycle life, and is reasonably cheap to produce.
Batteries are one of the most efficient ways to generate electrical energy. For comparison, a traditional power plant achieves electrical energy by turning chemical energy (lighting a combustible fuel) into thermal energy (boiling water to produce steam) into mechanical energy (turning a magnetic turbine). Complicated, right?
Batteries skip all those steps by converting chemical energy directly into electricity. This straightforward process means very little energy is lost.
To understand the impact of that, let’s contrast batteries and coal power plants. Coal power plants have an energy efficiency around 30%. That means they convert only 30% of the energy locked in the coal into electricity and lose the rest through all the energy transitions along the way.
A fuel-powered vehicle is even less efficient than that—only 12-30% of the energy from the fuel ultimately powers the car. Given sky-high gas prices, that’s depressing. In contrast, since batteries directly generate the electricity that powers a vehicle’s drive train, they’re a lot closer to a 100% energy conversion rate.
That said, although fuel offers an extremely inefficient energy conversion, it’s energy dense. Fuel has so much potential energy packed inside of it per liter that it’s still impactful at sub-30% efficiency (at least for now).
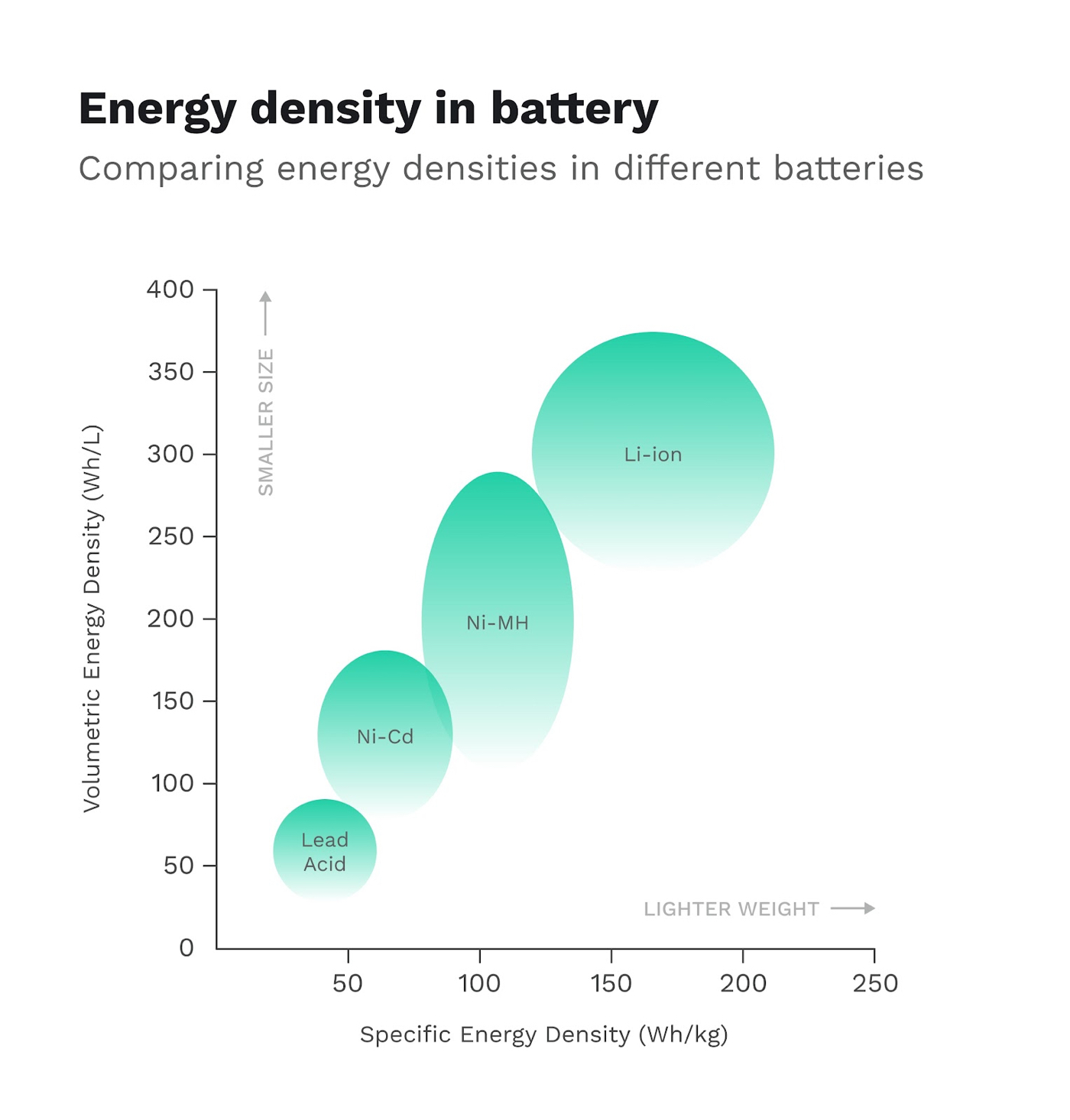
Batteries have the opposite profile. While they’re extremely efficient at energy conversion, they have an energy density that is 100 times less than that of gasoline. If you factor in that only 12-30% of the energy in gasoline actually goes into moving your vehicle, the comparison becomes closer. That said, batteries still lag behind.
Energy density is really just a matter of how many charged particles you can pack into a given volume. Lithium is already the smallest metal atom we have, and the most reactive at that. So to achieve even greater energy density from here on out will require some clever solutions and imagination from scientists.
Source: Dragonfly Energy
Our battery future: A renewable energy world needs better batteries
Battery design is more important than ever given climate change and sustainability. We have a long way to go to develop batteries that can cost-efficiently store the amount of renewable energy needed to electrify our infrastructure.
Many renewable energy sources like solar panels and wind turbines generate energy at sporadic times in line with the weather. If batteries can’t store this energy for crucial moments, it just goes to waste. According to Shirley Meng, a materials scientist at the University of Chicago who studies batteries, about 60% of the energy we produced in the last century went to waste.
As we discussed, another issue is that even top-of-the-line lithium batteries aren’t cost-competitive with gas-powered vehicles. Relying on just one battery chemistry, which depends on a dwindling supply of cobalt and lithium, is pushing prices up on batteries. As a result, we’re seeing a reversal of the impressive price decline from the past few decades.
Confronting these challenges has become a top priority for entrepreneurs, scientists, and governments around the world. The stakes are high, and battery technology needs to catch up with the realities of the 21st century.
It might just be that the only path forward for battery technology is a series of incremental gains. As Martin Eberhard pointed out, building a better battery has always been a matter of choosing between different trade-offs.
Do you value higher energy density or higher safety? More power output or a longer life cycle? For an excellent comparison of all the advantages and disadvantages of existing battery chemistries, take a look at the matrix compiled by Sarah Constantin.
What comes next for battery innovation?
Despite these challenges, there are still dozens of companies and researchers working on pushing out the frontier of battery capability. One option on the table is producing a battery with a lithium-metal anode. This would basically involve packing even more charged lithium particles into the battery to give it a higher energy density.
Though this idea has been around for decades, making such a dense mesh of highly reactive lithium has always been looked upon as dangerous. Early iterations of these batteries have failed and short-circuited as a result, but companies like QuantumScape are now confident they’re on the verge of new advancements for the technology.
Other impressive teams, like Natron Energy or Enerpoly, are primarily focused on creating battery alternatives for a world where we might become supply-constrained or priced out of using lithium-ion tech. They’re developing batteries which make use of sodium ions — the second lightest atomic metal after lithium — or rechargeable zinc-ion batteries, respectively. The resources required to build these batteries are more equally distributed around the world.
Alongside the great research being embarked on today, researchers like Shirley Meng of the University of Chicago are still convinced that rather than waiting around for an ideal one-size-fits-all cell, we will need to begin operating in a world where certain battery chemistries, along with their trade-offs, will be best suited for some use cases over others.
The world is on an exciting path forward as it searches for ever more efficient and renewable energy sources to power its activities. Batteries will continue being not only an important puzzle to solve in the journey ahead, but also one whose breakthroughs will engender a host of new and exciting technological applications, just as the lithium-ion has already done for us in the modern day.
Anna-Sofia Lesiv is a writer at venture capital firm Contrary, where she published an earlier version of this piece. She graduated from Stanford with a degree in economics, and has spent time at Bridgewater, Founders Fund, and 8VC.
The Only Subscription
You Need to
Stay at the
Edge of AI
The essential toolkit for those shaping the future
"This might be the best value you
can get from an AI subscription."
- Jay S.
Join 100,000+ leaders, builders, and innovators

Email address
Already have an account? Sign in
What is included in a subscription?
Daily insights from AI pioneers + early access to powerful AI tools


Comments
Don't have an account? Sign up!